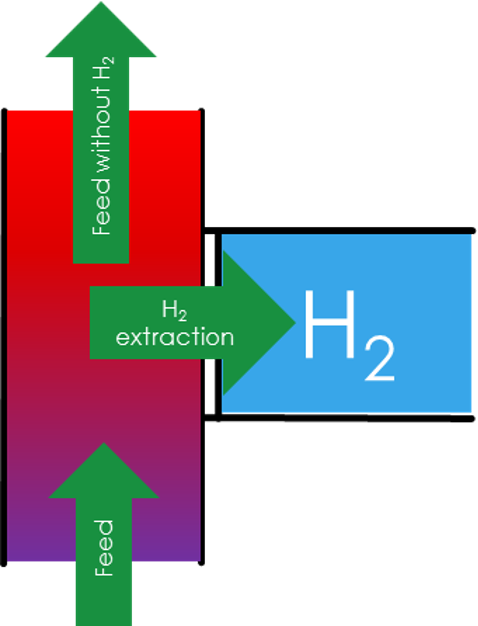
H2 Recovery
Based on the redoxpotential of hydrogen being 0V as well as the proton conductivity of the membrane, hydrogen can be filtered out of a gas stream. For example a process gas stream is contaminated with 2% hydrogen, which needs to be removed. With Proton Technologies H2 recovery system this 2% can be seperated from the main stream and concentrated over 99,999% purity (and higher). This is achieved by applying a voltage over Proton Technologies electrochemical cell. Hydrogen flows through a porous catalyst electrode which breaks the hydrogen molecule apart into protons and electrons. These protons will diffuse through Proton Technologies unique membrane and are recombined with electrons at the end of the membrane. This membrane is semipermeable, meaning protons can diffuse through but gas molecules can not, allowing to achieve very high purities.
As the system operates at <100°C and without palladium, it outperformes palladium purification systems in more than one way.
Possible applications:
- Gas chromatography
- SMR
- Glass making
- Semiconductor
- Metal treating
- Powder metallurgy